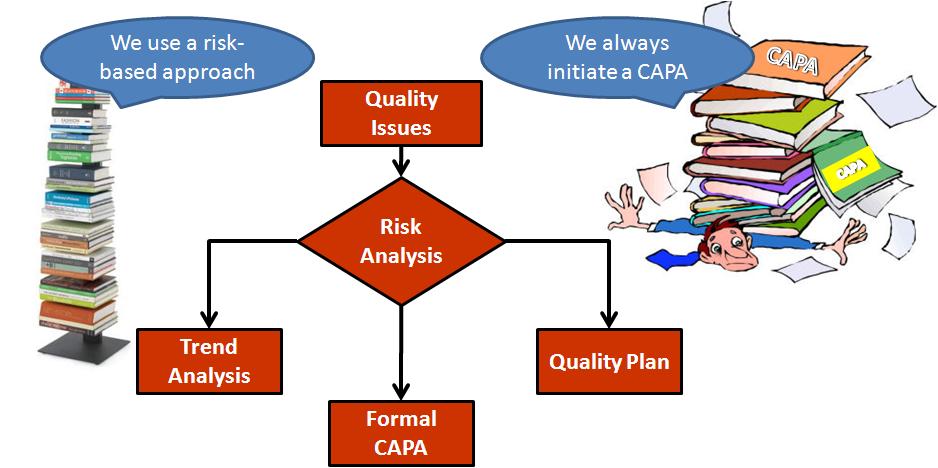
Principles of corrective Action and Preventive Action :CAPA: A Handbook for Quality Professionals in Medical device and Pharmaceutical Industries: MUCHEMU PhD, Dr DAVID NAKASALA: 9781790621101: Amazon.com: Books

CAPA Certification Assistance | Qualitas Compliance Raleigh | Medical Device Compliance Consulting, ISO 13485, Durham, Chapel Hill, RTP, Boston, Charlotte, Atlanta, Pittsburgh, Tampa, medical device risk management, fda submissions, gap analysis, CAPA


![Medical school building, University City, Madrid] | International Center of Photography Medical school building, University City, Madrid] | International Center of Photography](https://s3.amazonaws.com/icptmsdata/a/p/a/c/capa_robert_2010_86_156_470080_displaysize.jpg)